
Our New Opportunity Exploration Services help you identify, assess and check basic feasibility of opportunities whilst risk and investment is low.

We love design, engineering and technical problem solving challenges. Our medical device development services provide fresh thinking for new innovations, generate novel design concepts and proof of principle prototypes.

Our creative and engineering teams generate multiple design directions, CAD renders, and initial prototypes, always with manufacturability (DFM) in view

As one of the UK’s top medical device mechanical design agencies, our service is available at all stages of a project from concept to manufacture.
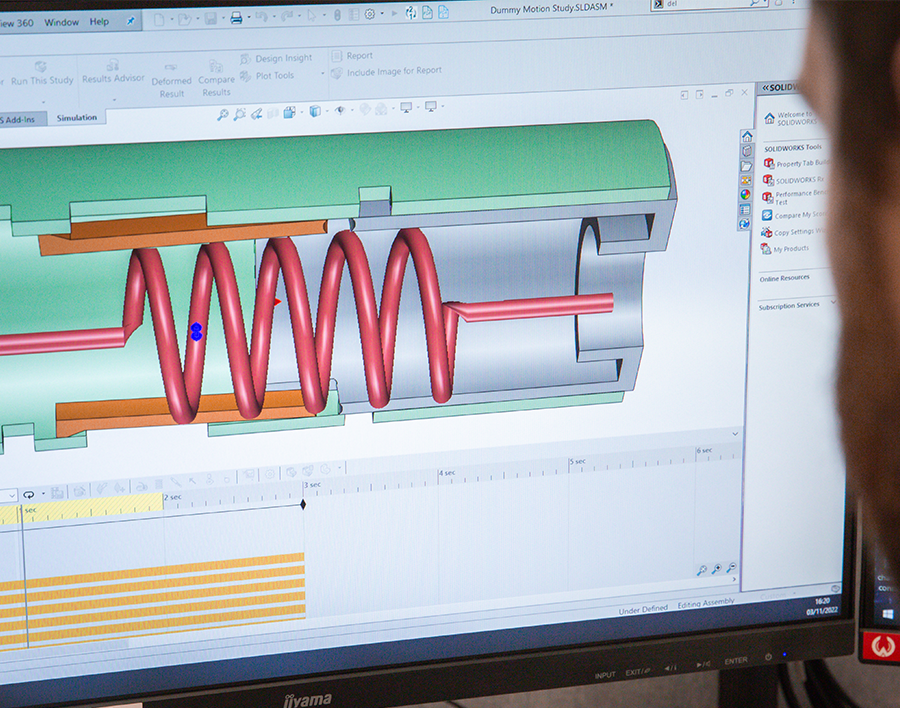
Our engineering design and Simulation services generate robustly designed products and devices that work as intended. We provide a range of ad-hoc services like FEA and Tolerance Analysis too.

Our human factors medical device development services help you understand your end-users’ capabilities, needs and desires, to improve overall user experience thus also adding commercial value to your product or device.

Our medical device development services cover the full electronic development process, including hardware and software, PCB design using Altium Designer for PCB prototypes through to production ready PCBs delivered directly by our approved suppliers

Our extensive industry partner network bring our designs to reality through a range of cost effective and high-quality manufacturing solutions.

Whether you would like to improve the sustainability of an existing product or, develop a novel device, we have the knowledge to guide and inform your development with lower environmental impact.

Our operation is certified to ISO:13485. Along with our in-house team, we’re partnered with world class specialists to provide additional regulatory guidance and support as part of our medical device development services.

If you’re looking for reliable, outcome-focused medical device development services, let’s talk. Whether you need full delivery or focused support in human factors, firmware, prototyping, or regulatory, we can scale to your needs.
Contact us for a consultation, quote or to explore how our approach can accelerate and de-risk your next medical device project.
Get in touch >At Haughton Design, our working approach is built on flexibility, traceability, and collaboration. We understand that every project is unique, so our medical device development services adapt to fit your preferred way of working — whether that means full turnkey delivery or targeted support within your own systems.
We offer two main collaboration models:
Turnkey Projects
For clients seeking a complete, end-to-end solution, we manage the entire process under our ISO 13485 and ISO 9001-certified Quality Management System. This ensures every stage — from concept and feasibility through detailed design, verification, validation, and transfer to manufacture — is fully documented and compliant. Our multidisciplinary team handles all aspects of design, engineering, usability, and risk management, providing complete traceability and regulatory readiness.
Bolt-On Support
When additional expertise or capacity is needed, we integrate seamlessly into your existing processes, acting as an extension of your team. We work within your documentation, quality procedures, and risk frameworks, maintaining consistency and compliance while accelerating delivery. This model offers flexible access to specialist capabilities across mechanical, usability, electronic, and human factors engineering.
Whichever model you choose, our approach combines innovation with control. Using a clear, visual workflow, we map responsibilities, milestones, and traceability throughout development. This structured yet agile process ensures visibility, accountability, and efficient decision-making — helping you progress from concept to compliant, manufacturable product with reduced risk and greater confidence in quality and performance.